Q. I have a heat transfer problem, which was raised by a group of students. The problem is concerned with the design of a cooling reservoir that is part of their final year project. Basically, they asked me how to calculate the temperature at time, t, of the water in a reservoir. The reservoir receives a constant water supply, say, 100 m^3/hour at a temperature of, say, 45 C. The reservoir has an initial volume of water, for example, 100 m^3 at a temperature, say 10 C. If we assume that the reservoir has a size of 1m x 30m x 30m (water exposed area is 900 m^2), about 8 hours are required to fill the reservoir and then the excess of water will be over flown. The problem is how to estimate the water temperature, Tt, at a time, t. In other words, to check the time required for the water in the reservoir, to cool down to the ambient temperate (8 C). I would be very grateful if you can help me with this problem. Kind regards.
R. This problem is an example of a non steady open system. Let the system boundary enclose only the water that is inside the reservoir. The underlying principle is that the net rate at which energy is transferred and transported into the system equals the rate of change of the energy of the system.
Internal energy is transported into and out of the system with the water flows and is lost from the system as heat transfer to the surroundings. When the relevant simplifying assumptions are made the situation is described by a first order differential equation in temperature. This can be integrated from the initial temperature of the water in the reservoir at time zero to give the temperature of the water at any future time. The flow rate of water into the system is steady. There is no flow out of the system until the overflow level is reached. Thereafter the flow rate out of the system is steady and equals the flow rate in. After a finite time the temperature of the water leaving will be within a specified tolerance of the eventual (infinite time) steady state value.
You may or may not agree with the simplifying assumptions mentioned here. They are your responsibility and you can adapt them as appropriate.
The non flow energy equation for the system can be written as follows:
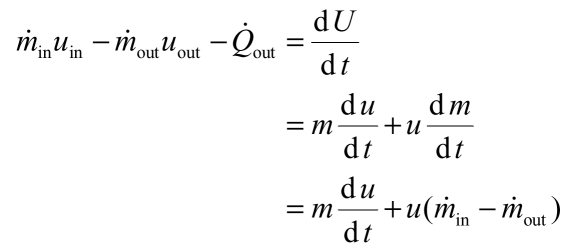
The following expressions describe the specific internal energy of the water, the rate of heat transfer out of the system to the surroundings and the mass of water in the system:
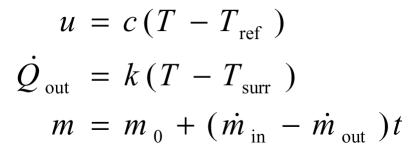
The differential equation describing the rate of change of the temperature reduces to:
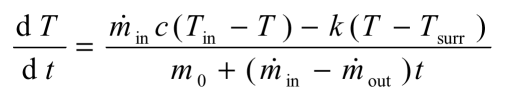
|
|
Leo Nest, ULFC
<< Back to Ask Leo About Thermo
© Jim McGovern, 2001 – 2005
Send questions or comments about this site to Webmaster.