Q. My classmates and I have heard that you have a special interest in the concept of exergy in thermodynamics. As it is we find the sign convention for heat and work quite confusing, but the situation gets even more complicated in exergy analysis. Can you please let us have your views on this matter.
R. You are perfectly entitled to be confused. There is a need for clear thinking here, taking one step at a time. Thermodynamics, like most of human knowledge, has not developed in a clear logical progression. Some confusing remnants of its history and some happenstance are still embedded in its terminology and in the way the theory is currently applied. Never mind. Be patient. I'm delighted to give you my viewpoint and I hope you find it helpful.
There are two sign conventions in common use. As you will see, I have my own preference!
Firstly, a convention is just that: it is there to be overridden or ignored at will. All engineers and thermodynamicists do this as a matter of course; e.g. by referring to the ‘heat rejection’ from the condenser of a power plant, rather than the ‘negative heat input!’ Of course, conventions are there to simplify life and most people are happy to fall in line.
Two main sign conventions are used in thermodynamics. The first is still the most common in the English-speaking world and probably dates back to the introduction of steam engines. In that era, engineers were particularly interested in heat engines for pumping water or driving machinery. The desired output was work and the required input was heat. It made sense to describe both as positive quantities. It was probably because of this that the first convention was established: in this convention heat transfer into a system and work output from a system are both taken as positive. Therefore, any heat transfer out of the system and any work input are both negative. The ‘Positive heat in, positive work out’ convention is illustrated below.
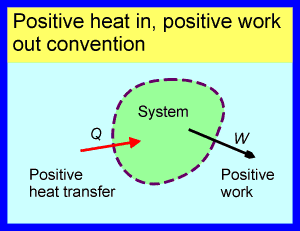
The second sign convention, which I favour, has a large and growing number of advocates in the English-speaking world and is the most common in the non-English-speaking world. According to this convention heat and work are both taken as positive when their direction is into the system of interest. Heat and work are negative when they are out of the system of interest. The ‘Positive heat in, positive work in’ convention is illustrated below.
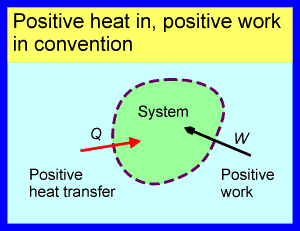
In my opinion the second convention is the most consistent, as all transfers or interactions at the boundary can be treated in the same way: they are taken as positive when inwards and negative when outwards. For instance, any energy input to a system is taken as positive irrespective of whether it is energy transfer as heat, energy transfer as work, or energy transport with substance that crosses the boundary. Thus the second convention can be thought of as a subset of the ‘Positive in’ convention, which is completely general as it is not limited to just heat and work interactions. The ‘Positive in’ convention is illustrated below.
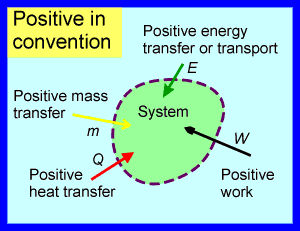
The same consistent ‘Positive in’ convention can be used for all quantities that can enter or leave a system: e.g. mass, moles of substance, kinetic energy, entropy and Gibbs free energy (a more specialised thermodynamic property).
The ‘Positive heat in, positive work out’ convention complicates the teaching and application of the first law of thermodynamics. This is perhaps best explained by taking a simple example from an unrelated area: suppose we wish to teach the concept of ‘the change in the number of sweets in a bag’ to children who have already mastered positive and negative numbers. We use a bag containing red sweets and yellow sweets and we also have a pile of red sweets and yellow sweets on the table. We could use either of the following methods to define ‘the change in the number of sweets in the bag’:
Method 1
change = (red sweets added) - (yellow sweets removed)
Method 2
change = (sweets added)
Both methods are valid and are equivalent in the algebraic sense, but method 2 is more straightforward. Method 2 here is analogous to using the ‘Positive in’ convention to explain the first law of thermodynamics. Energy can enter (or leave) a closed system as heat or work. It is perfectly valid to describe the increase in energy of a system as the input of heat minus the output of work, but it is not straightforward.
The exergy of a system is the potential for the doing of mechanical work by the interaction of the system with a specified, all-enclosing, equilibrium environment. The area of exergy analysis (or second law analysis) is often considered an advanced topic and may, therefore, be omitted from introductory engineering thermodynamics courses, which is a pity. In exergy analysis the use of the ‘Positive heat in, positive work out’ convention is counter-intuitive and confusing. Why? The ‘Positive in’ convention, on the other hand, is fully intuitive. I consider this a very strong reason for using the ‘Positive in’ convention in thermodynamics generally.
Leo Nest, ULFC
<< Back to Ask Leo About Thermo
© Jim McGovern, 2001 – 2005
Send questions or comments about this site to Webmaster.